Cosmetic regulation in Mexico

In publication #008 of KosmetikOn Education we deal in depth with cosmetic regulations in Mexico. We start with the current cosmetic standards followed by their definition and annexes. We continue with the labelling section. Finally, we finish with the notification.
Cosmetic standards and regulations in force in México
In Mexico, cosmetic products are regulated by two Official Mexican Standards, an Agreement determining the substances prohibited and restricted in the manufacture of cosmetic products [1] and the General Health Law [2]. The Official Mexican Standard 141 for the labelling (Labeling for pre-packaged cosmetic products, Sanitary and commercial labeling) [3] adds information on the label of the products and also some definitions.
These lists make it possible to know the substances that may be used when manufacturing a product. Articles 234 and 245 of the General Health Law add two lists of substances also prohibited in cosmetics.
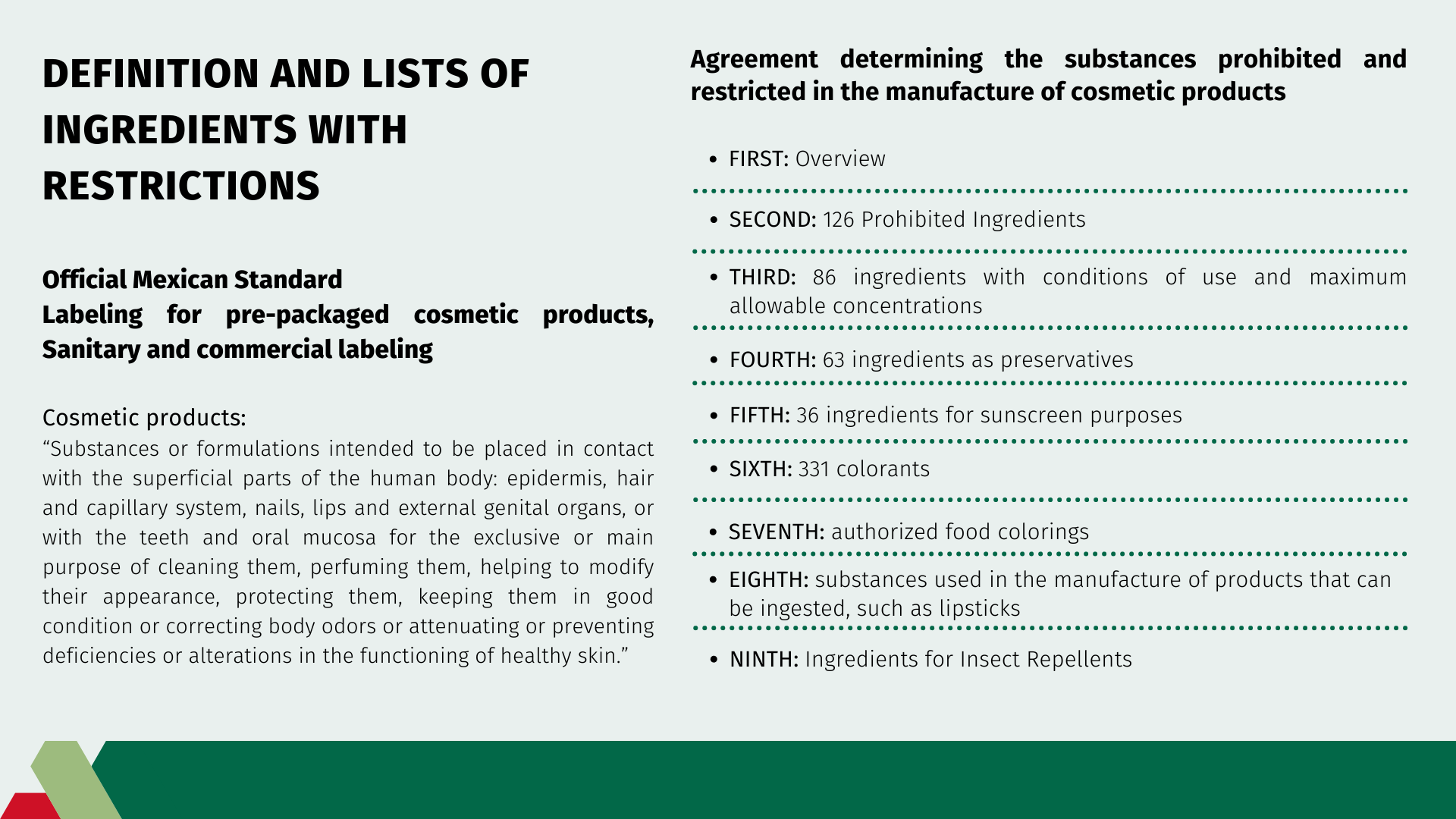
Definition and lists of ingredients with restrictions
The Agreement determines the lists of ingredients in the manufacture of perfumery and beauty products. The summary of this document is:
FIRST: Overview
SECOND: 126 Prohibited Ingredients
THIRD: 86 ingredients with conditions of use and maximum allowable concentrations.
FOURTH: 63 ingredients as preservatives
FIFTH: 36 ingredients for sunscreen purposes
SIXTH: 331 colorants
SEVENTH: authorized food colorings
EIGHTH: substances used in the manufacture of products that can be ingested, such as lipsticks
NINTH: Ingredients for Insect Repellents
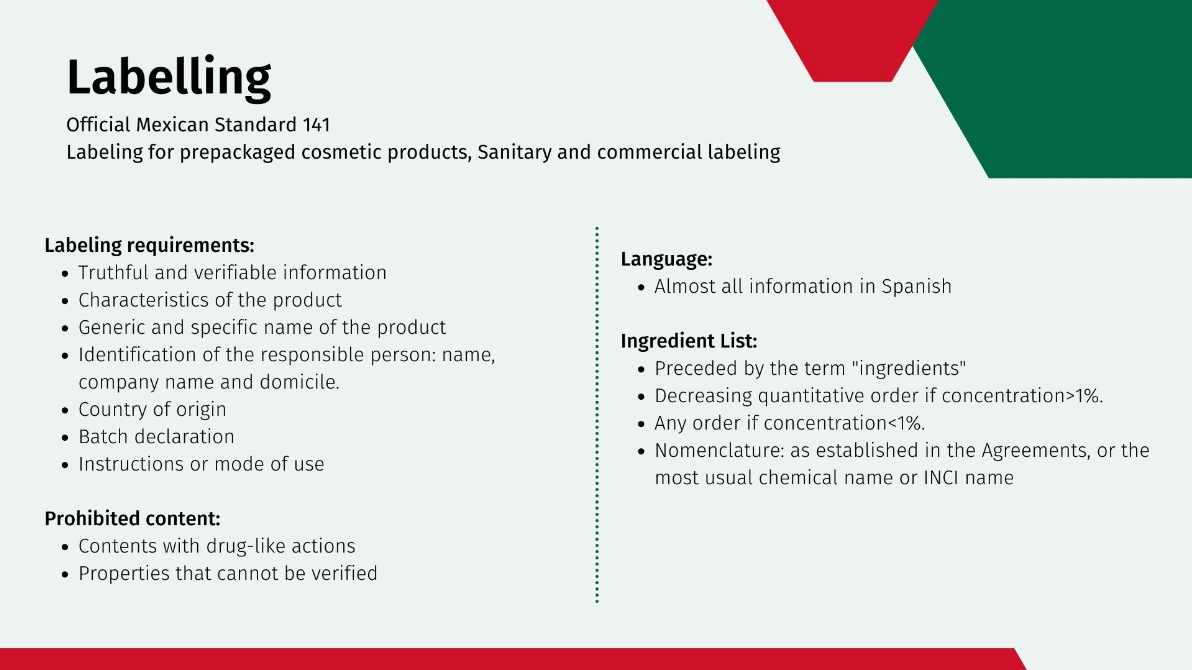
Labelling
Mexican Standard 141 contains labeling information for prepackaged cosmetic products.
First, the information contained in the label must be in Spanish. For the nomenclature of ingredients, any of those established in the Agreements, or the most usual chemical name or the name as it appears in the International Nomenclature of Cosmetic Ingredients (INCI), may be used at the choice of the manufacturer. Fragrances and flavors may be designated by the generic name. Materials of botanical origin should be designated by the scientific name of the plant, the common name of the plant being optional.
This standard defines the labeling requirements:
Information presented to the consumer must be truthful and verifiable
Characteristics of the product
Generic and specific name of the product
Identification of the person responsible for the product: name, denomination or company name and domicile
Country of origin of the product
Batch declaration
Instructions or mode of use
There are also prohibited contents on labels:
Contents that have actions characteristic of medicinal products
Claims that cannot be verified
The ingredient list of the formula shall appear on the label preceded by the term ingredients. This list shall be in descending quantitative order of those ingredients in concentrations greater than 1% followed by those ingredients in concentrations less than or equal to 1% which may be listed in any order.
For ingredients that pose health risks according to the Secretary, the precautionary statements associated with these ingredients must be in Spanish. There are also precautionary statements that must be included in the labelling depending on the type of product (e.g. deodorants, dyes, bleaches, nail hardeners, sunscreens, talcum powder...).
Other Mexican standards, such as 002 and 030, complete the information required for a product label. If the cosmetic product is too small for all the information to be put on a label, the information shall be indicated in an accompanying leaflet.
Notification
Cosmetic products must also comply with the requirements of COFEPRIS [4]. This national authority for the protection against health risks requires a Notice of Performance for cosmetic products. The Notice of Operation must identify all steps involved in the manufacturing process of cosmetic products (e.g. storage, marketing, manufacturing, export, packaging, etc.).
References
Agreement determining the substances prohibited and restricted in the manufacture of cosmetic products. available at https://dof.gob.mx/nota_detalle.php?codigo=5143790&fecha=21/05/2010#gsc.tab=0 (July. 2022)
General Health Law available at https://www.diputados.gob.mx/LeyesBiblio/ref/lgs.htm (July. 2022)
Official Mexican Standard 141 for the labelling. Labeling for pre-packaged cosmetic products, Sanitary and commercial labeling available at https://dof.gob.mx/nota_detalle.php?codigo=5269348&fecha=19/09/2012#gsc.tab=0 (July. 2022)
Government of Mexique. COFEPRIS available at https://www.gob.mx/cofepris/acciones-y-programas/aviso-de-funcionamiento-de-responsable-sanitario-y-de-modificacion-o-baja (July. 2022)