Cosmetic regulation in the US

In publication #004 of KosmetikOn Education we deal in depth with cosmetic regulations in the US. We start with the two most important authorities which regulate the cosmetics market, and their definiton of a cosmetic product. We continue with the particular case of dyes. Finally, we finish with the sections of adulteration, labelling and the Voluntary Cosmetic Registration Program.
Cosmetic standards and regulations in force
Cosmetics marketed in the United States have to follow the two most important laws related to the Federal Food, Drug and Cosmetic Act (FD&C) [1] and the Fair Packaging and Labeling Act (FPLA) [2]. The FDA regulates cosmetics in accordance with these laws. In the United States, Congress passes federal laws. To make the laws effective on a day-to-day basis, Congress authorizes certain government agencies, such as the FDA, to create regulations. Cosmetics are not FDA-Aproved, but are FDA-Regulated. Cosmetics have to follow other State Cosmetics Policies, being California one of the more actives in it.
Definition
First, the FD&C regulation defines cosmetics by their intended use as articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body...for cleansing, beautifying, promoting attractiveness, or altering the appearance.
Included in this definition are products such as skin moisturizers, perfumes, lipsticks, fingernail polishes, eye and facial makeup preparations, shampoos, permanent waves, hair colors, toothpastes, and deodorants, as well as any material intended for use as a component of a cosmetic product. The definition of a cosmetic excludes soap from the definition of a cosmetic.

FDA
The law does not require cosmetic products and ingredients other than colorants to have FDA [3] approval before entering the market. However, FDA may take enforcement action against products offered on the market that do not comply with the law or against companies or individuals who violate the law. FDA may inspect establishments that manufacture cosmetics to ensure the safety of the cosmetic product and to determine whether cosmetics are adulterated or misbranded under the FD&C Act or the FPLA.
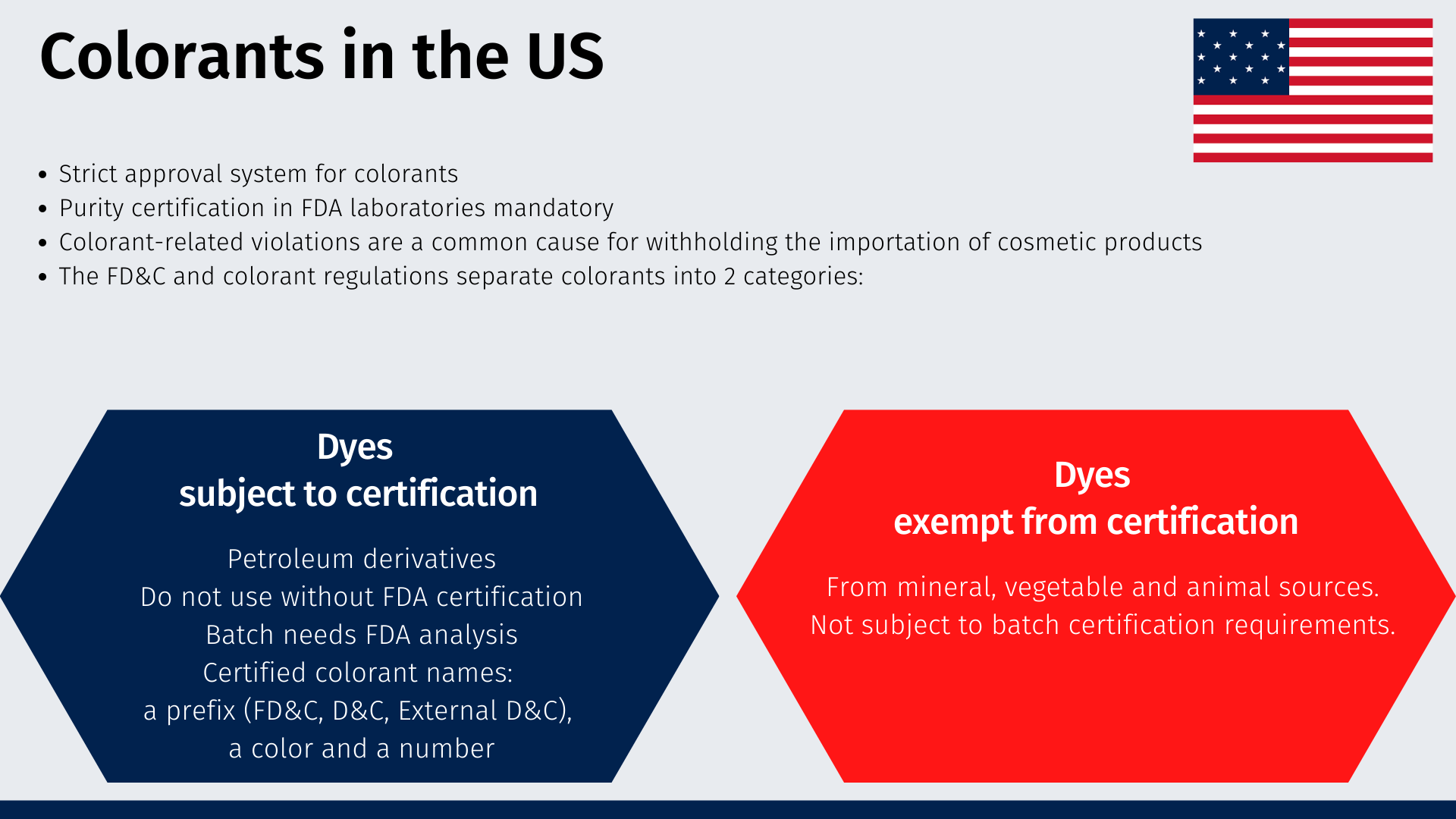
Colorants
Colorants are subject to a strict approval system under U.S. law. Before marketing a product containing a colorant in the United States, it is essential to determine whether the colorant is approved for its intended use. Various colorants must be certified for purity in FDA laboratories if they are to be used legally in a product marketed in the United States. Colorant violations are a common cause for withholding the importation of cosmetic products entering the country.
The Federal Food, Drug, and Cosmetic Act, and the colorant regulations [4] separate approved colorants into two main categories: those subject to certification and those exempt from certification.
- Colorants subject to certification: These colorants are derived primarily from petroleum. These colorants must not be used unless FDA certifies that the lot in question passed analysis of its components and purity in FDA laboratories. If the lot is not FDA certified, do not use it. Certified colorants generally have three-part names: a prefix (FD&C, D&C, External D&C), a color and a number.
- Colorants exempt from certification: These colorants are obtained mainly from mineral, vegetable and animal sources. They are not subject to batch certification requirements.
- If the product contains colorants, in accordance with the law it must meet the requirements for the following:
- FDA approval
- Certification for a quantity of colorants
- Identity and specifications: All colorants must meet the identity and specification requirements set forth in the Code of Federal Regulations
- Use and restrictions: Colorants must only be used for the intended uses set forth in the regulations pertaining to them. The regulation also specifies other restrictions for certain colorants, such as the maximum concentration allowed in the final product
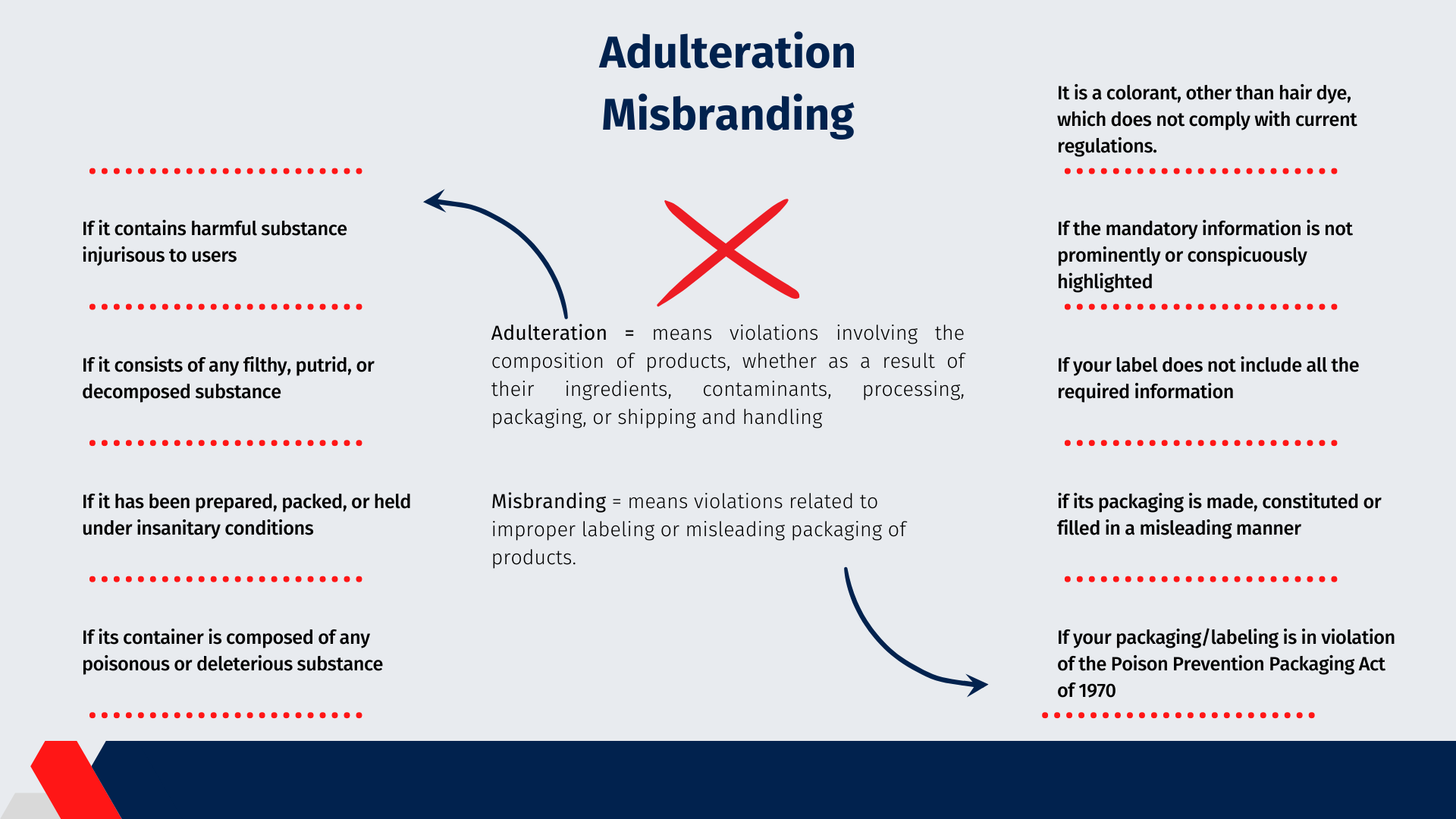
Alduteration
The FD&C Act prohibits the marketing of cosmetics that are adulterated or misbranded in interstate commerce. Adulteration means violations involving the composition of products, whether as a result of their ingredients, contaminants, processing, packaging, or shipping and handling. Instances of adulteration are:
If it bears or contains any poisonous or deleterious substance which may render it injurious to users under the conditions of use prescribed in the labeling thereof, or, under such conditions of use that are customary or usual
If it consists in whole or in part of any filthy, putrid, or decomposed substance
If it has been prepared, packed, or held under insanitary conditions whereby it may have become contaminated with filth, or whereby it may have been rendered injurious to health
If its container is composed, in whole or in part, of any poisonous or deleterious substance which may render the contents injurious to health
Misbranding means violations related to improper labeling or misleading packaging of products. According to the FD&C Act, a cosmetic is considered to present brand adulteration in the following cases:
False or misleading information
Lack of required information
Conspicuousness and readability of required information
Misleading packaging
Improper packaging and labeling of color additives
Conspicuousness and readability of required information
Misleading packaging
Improper packaging and labeling of color additives
Deficiencies where the Poison Prevention Packaging Act requires special packaging
If your product or your company violates these laws in the marketplace, the FDA may take action against the person(s) marketing such products.
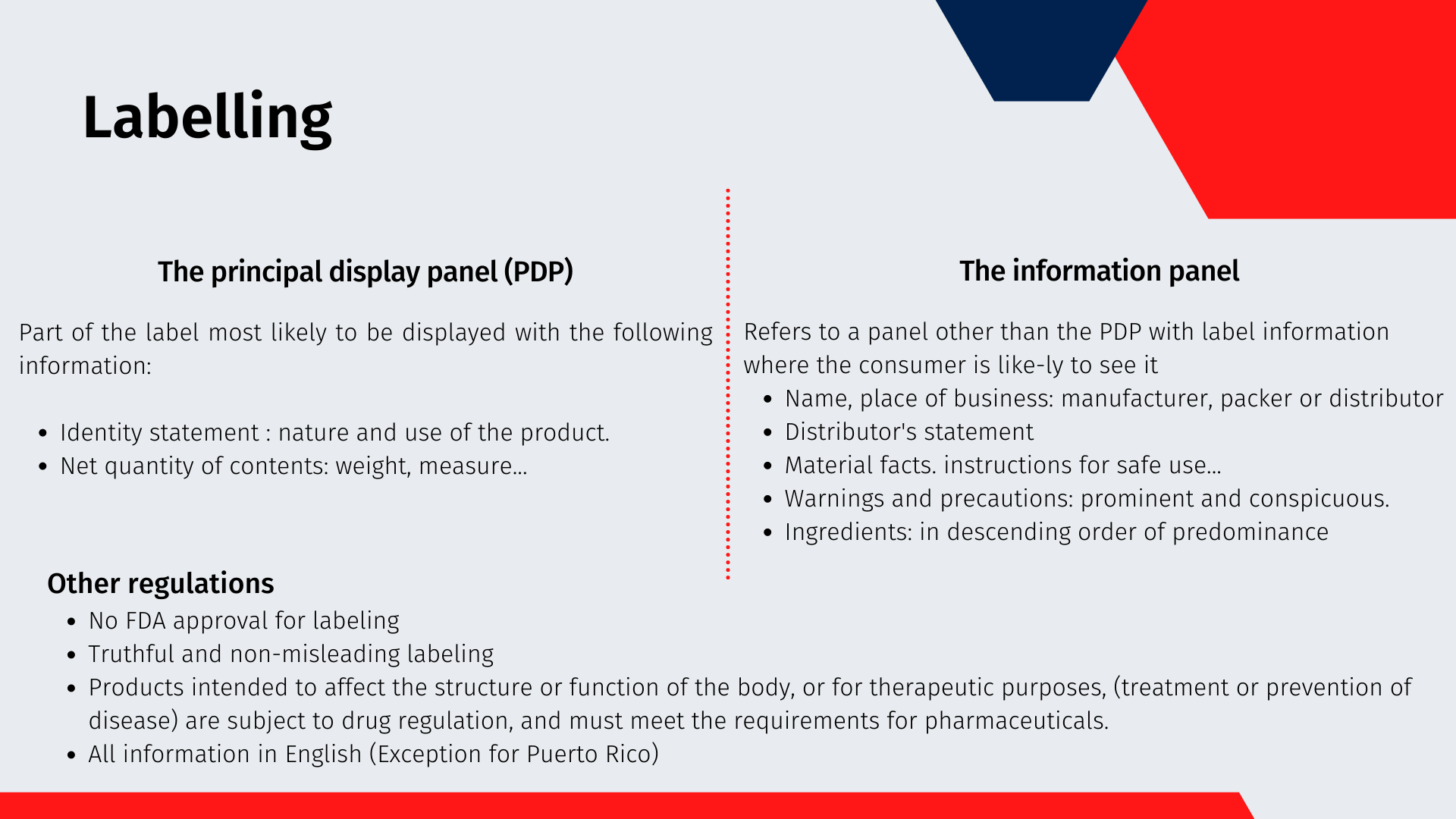
Labelling
The law does not require cosmetic labeling to have FDA approval before cosmetic products go on the market, and FDA does not have a list of approved or accepted claims for cosmetics. However, there are limits that apply to cosmetic labeling claims. Cosmetic product labeling must be truthful and not misleading. Products intended to affect the structure or function of the body, or for therapeutic purposes, such as the treatment or prevention of disease, are subject to drug regulation, and must meet the requirements for pharmaceuticals.
Regarding the language, all labeling information required by law must be in English. The only exception to this rule is for products distributed only in a U.S. territory where a different language predominates, such as Puerto Rico.
The regulations establish two different parts of the packaging where different information is displayed. The principal display panel: The part of a panel that is most likely to be shown or examined under customary conditions of display for retail sale. The following information must appear:
An identity statement: nature and use of the product
Net quantity of contents: in terms of weight, measure, numerical count or a combination of numerical count and weight or measure
The information panel: refers to a panel other than the PDP that can accommodate label information where the consumer is likely to see it. Since the information must be prominent and conspicuous, the bottom of the package is generally not acceptable for placement of required information, such as the cosmetic ingredient declaration. The following information must appear on this panel:
Name and place of business: the manufacturer, packer or distributor
Distributor statement: If the name and address are not those of the manufacturer, the label should read Manufactured for... or Distributed by...
Material facts: An example is directions for safe use, if a product could be unsafe if used incorrectly
Warnings and cautions: These must be prominent and conspicuous
Ingredients: Must be in descending order of predominance. And the ingredients are named following an International “Harmonization” of Ingredient Names edited on the Dictionary and Handbook by the Personal Care Products Council (PCPC) [5]
FDA's Voluntary Cosmetic Registration Program (VCRP)
FDA's Voluntary Cosmetic Registration Program (VCRP) is a reporting system for use by manufacturers, packers, and distributors of cosmetic products that are in commercial distribution in the United States. It does not apply to products that are not for sale, such as hotel samples, free gifts, or cosmetic products you make in your home to give to your friends. There are two parts to the VCRP filing, you may participate in both parts of the program or only one part.
Registering cosmetic manufacturing and/or packaging establishments (Form FDA 251): This form requires the following information: Type of Establishment, Establishment Name, Parent Company Name (if any), Address, Address Location, Owner or Operator of the Facility, Other Business Trading Names, Establishment Authorized Individual…
Cosmetic Product Ingredient Statements (CPIS) (Form FDA 2512): This form requires the following information: Labeler Type of Business, Labeler Name and Address, Provide Manufacturer/Contract Manufacturer Information, Provide Packer Information, Commercial Distribution, Product Information, Ingredients…
If the manufacturer of a cosmetic product submits the product formulation to the VCRP, FDA can alert the company if it inadvertently uses an unauthorized colorant or other prohibited or restricted ingredient. That way, manufacturers can correct their formulations before trying to market them in the United States and avoid the risk of having their products detained and denied entry into the United States because of the prohibited ingredient.

References
The United States Code. Federal Food, Drug and Cosmetic Act available at http://uscode.house.gov/browse/prelim@title21/chapter9&edition=prelim (July. 2022)
U.S. Food and Drug Administration. Fair Packaging and Labeling Act available at https://wayback.archive-it.org/7993/20170722051950/https:/www.fda.gov/RegulatoryInformation/LawsEnforcedbyFDA/ucm148722.htm (July. 2022)
U.S. Food and Drug Administration available at https://www.fda.gov/ (July. 2022)
Code of Federal Regulation. Colorant Regulations available at https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-70 (July. 2022)
Personal Care Products Council. INCI available at https://www.personalcarecouncil.org/resources/inci/ (July. 2022)