COSMETIC REGULATION IN THE EURASIAN ECONOMIC UNION (EAEU)

In publication #009 of KosmetikOn Education we deal in depth with cosmetic regulations in The Eurasian Economic Community. First of all, we present you the member-states of The Eurasian Economic Community and the current cosmetic standards. We continue with their definition, their annexes and the specific requirements. Finally, we finish with the labelling section and all the information you will need to register your cosmetic product.
Cosmetic standards and regulations in force
Russia, Kazakhstan, Belarus, Armenia and Kyrgyzstan have formed the Eurasian Economic Union (EAEU). This Customs Union has adopted a common cosmetics legislation: the Technical Regulation on the Safety of Cosmetic Products (CU TR 009/2011) [1] that came into force on 1 July 2012. On March 19, 2019, a new amendment to the Technical Regulation "On safety of perfume and cosmetic products" was adopted by Decision No. 32 of the Council. With this Amendment, the requirements have been harmonized with the EU Cosmetics Regulation. Therefore, a cosmetic product must follow the EU standards concerning the list of prohibited and restricted ingredients, and also the lists of authorized colorants, preservatives and UV filters.

Definition and lists of ingredients with restrictions
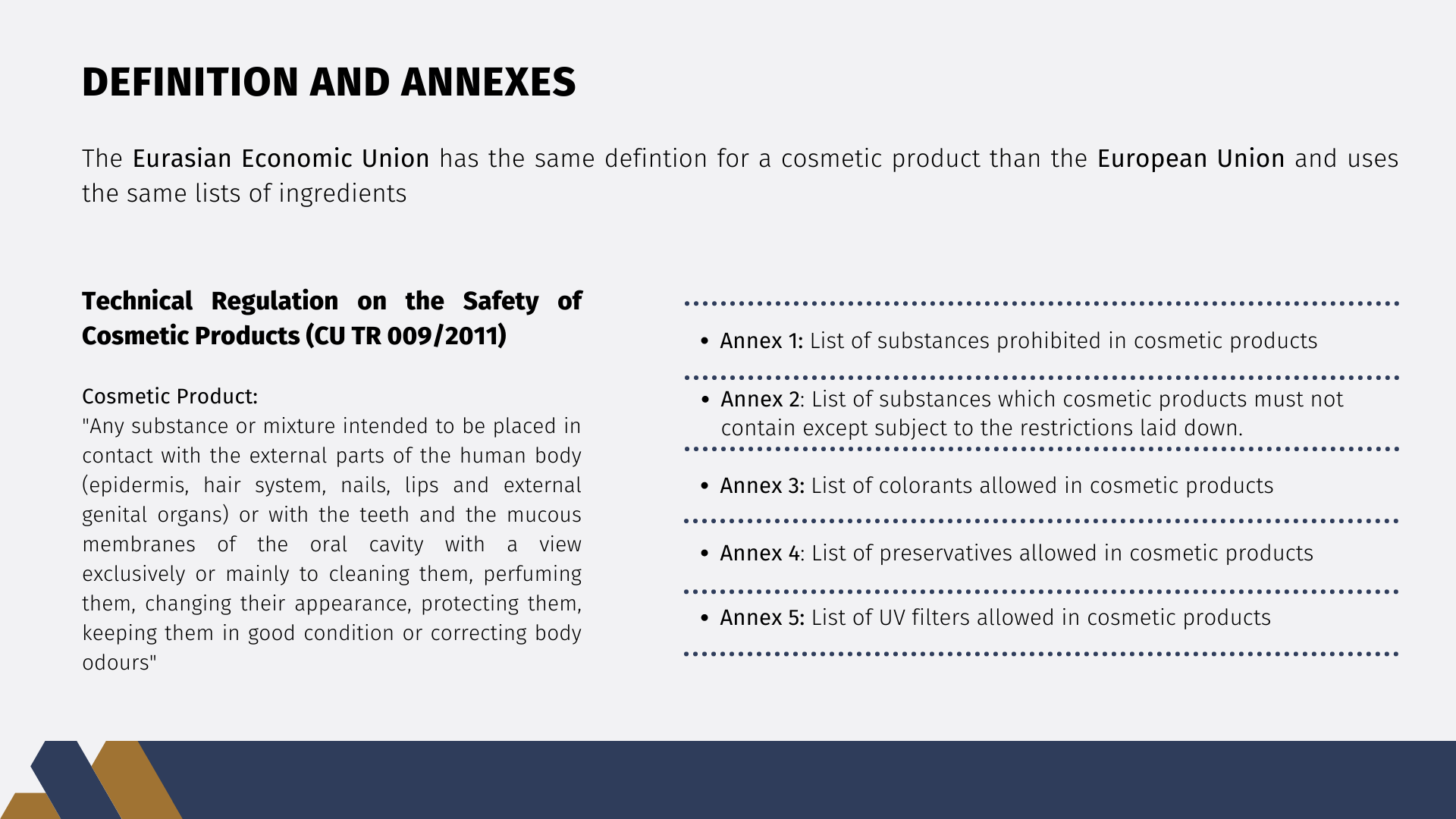
The definition of cosmetic products in the EAEU is the same as the EU definition: "cosmetic product means any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odours".
The EAEU also uses the lists of ingredients present in the annexes of the European Regulation:
- Annex 1: List of substances prohibited in cosmetic products
- Annex 2: List of substances which cosmetic products must not contain except subject to the restrictions laid down
- Annex 3: List of colorants allowed in cosmetic products
- Annex 4: List of preservatives allowed in cosmetic products
- Annex 5: List of UV filters allowed in cosmetic products
The Customs Union provide specific requirements for the perfume and cosmetic product in the other annexes:
- Annex 6: Requirements pH value for the perfume and cosmetic products
- Annex 7: Microbiological Safety Performance Indicators perfume and cosmetic products
- Annex 8: Requirements for Toxicological Data perfume and cosmetic products
- Annex 9: Requirements for clinical (clinical and laboratory) Index of perfume and cosmetic products
- Annex 10: Requirements for clinical indicators of oral hygiene
- Annex 11: Symbol indicates the presence of additional information about the perfume and cosmetic products
- Annex 12: List perfumes and cosmetic products subject to state registration

Labelling
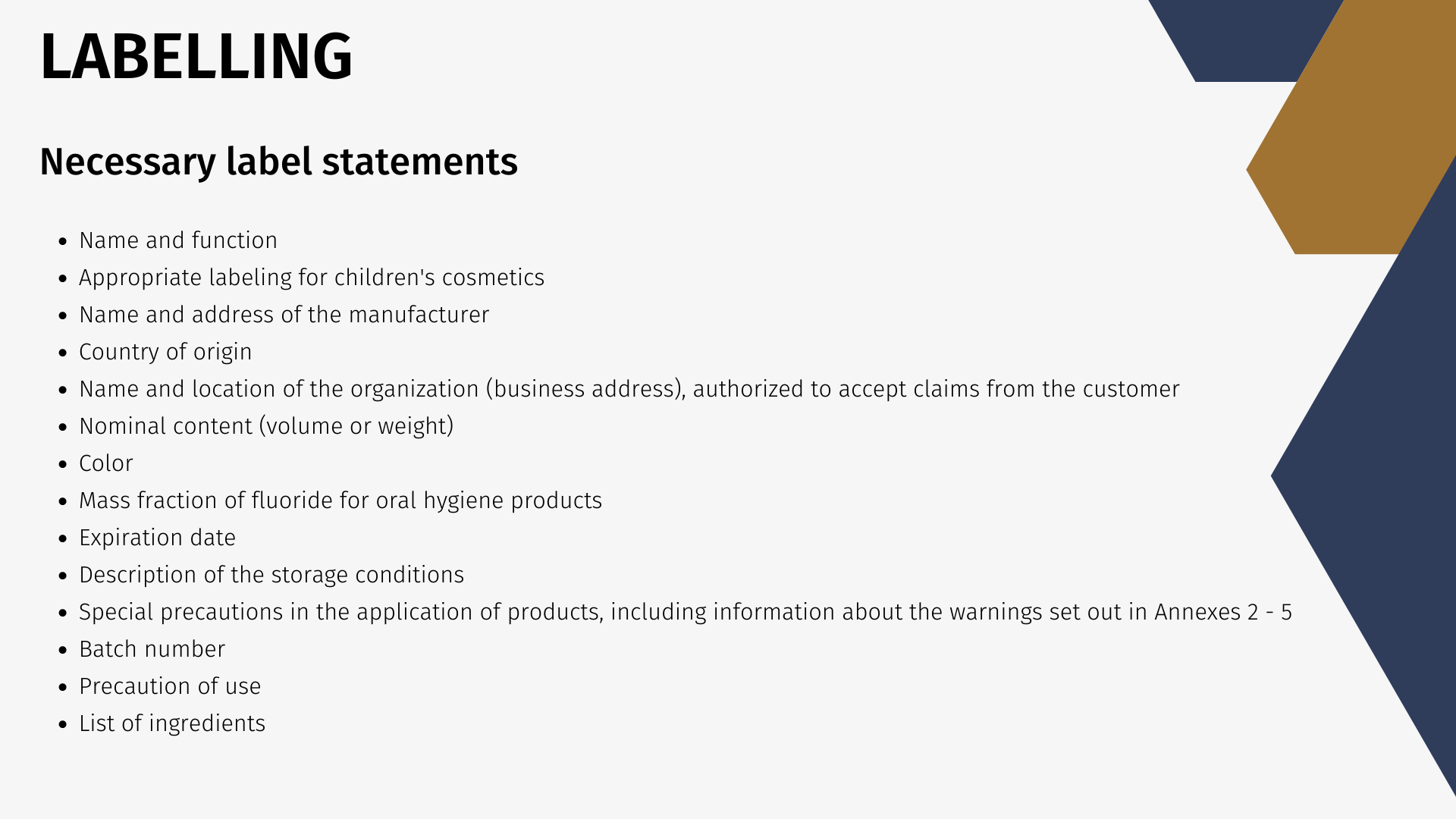
Chapter 9 of the Technical Regulation deals with the labeling requirements for perfumes and cosmetics. The label should contain the following information:
- Name of the cosmetic
- Function of the product, if it does not follow from product names
- Cosmetics, designed for children, should have the appropriate information in labeling
- Name and address of the manufacturer
- Country of origin
- Name and location of the organization (business address), authorized by the manufacturer to accept claims from the customer
- Nominal content (volume or weight) of the product (except for perfumes and cosmetics nominal weight of less than 5 grams, or a nominal capacity of less than 5 ml, or samples)
- Color and / or tone (for color cosmetics and colorants)
- Mass fraction of fluoride (% or mg / kg or ppm) for oral hygiene products containing fluorine compounds
- Expiration date: date of manufacture (month, year) and shelf life (months, years) or the words "best before" (month, year) or "use up" (month, year)
- Description of the storage conditions if these conditions are different from the standard
- Special precautions (if necessary) in the application of products, including information about the warnings set out in Annexes 2 - 5 of this technical regulation
- Batch number or a special code allowing identification of the cosmetics
- Information on how to apply perfume and cosmetic products, the lack of which can lead to misuse customer perfumes and cosmetics
- List of ingredients
This information shall be provided in the state (s) language (s) of the CU Member States, in which the sale of perfumes and cosmetic products. Only the name of the manufacturer, the manufacturer's location and product name can be written using the letters of the Latin alphabet.
The list of ingredients should be preceded by the title "Ingredients" or "Composition", and established in order of decreasing mass fraction in the formula. They can use the official language (s) of states members or the International Nomenclature of Cosmetic Ingredients (INCI) using the Latin alphabet. If the composition includes ingredients (N 67 92), Annex 2, and their content is higher than the concentration of 0.01% for rinse-off products, 0.001% for leave-on products, they should be listed in the lineup. Ingredients in concentrations of less than 1% may be listed in any order after those ingredients whose concentrations over 1%. Dyes may be listed in any order after the other ingredients in accordance with the color index. Ingredients present in the form of nanomaterials shall be clearly indicated in the list of ingredients showing after their name in brackets include "nano".
If all the information is not on the label, the symbol present in the annex 11 should be on the product to indicate that there is an accompanying leaflet.

Notification
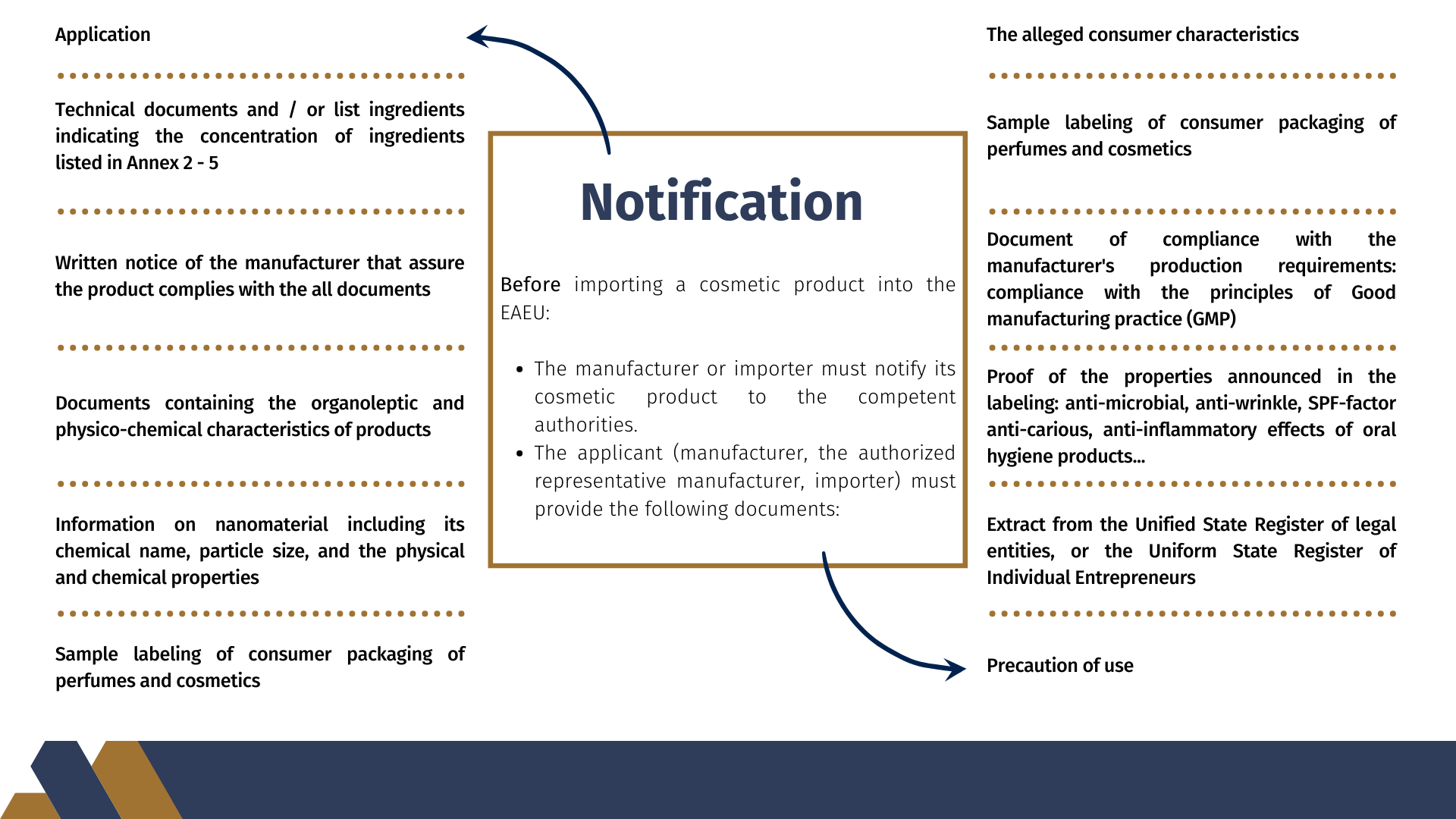
Before importing a cosmetic product into the EAEU, the manufacturer or importer must notify its cosmetic product to the competent authorities. The applicant (manufacturer, the authorized representative manufacturer, importer) must provide the following documents:
- Filled up Application
- Copies of the documents, according to which the products are manufactured (technical documents and / or list ingredients that make up the perfume and cosmetic products, indicating the concentration of ingredients listed in Annex 2 - 5), certified by the applicant.
- Written notice of the manufacturer that assure the product complies with the all documents. As the notice is received by the manufacturer's certificate of quality products, certified by the manufacturer.
- Copies of the documents containing the organoleptic and physico-chemical characteristics of products certified by the applicant.
- Information on nanomaterial including its chemical name, particle size, and the physical and chemical properties
- Sample labeling of consumer packaging of perfumes and cosmetics
- Annotation containing the alleged consumer characteristics (unless the manufacturer states in their product labeling)
- Special precautions (if necessary) in the application of products and information on how to apply perfume and cosmetic products, the lack of which can lead to misuse customer perfumes and cosmetics
- Protocols (tests), or scientific reports, or expert advice
- Document of compliance with the manufacturer's production requirements: compliance with the principles of GMP, or a certificate of quality management system, or a certificate conformity of production of perfume and cosmetics products to the principles of good manufacturing practice (GMP)
- Proof of the properties of perfumes and cosmetics, announced in the labeling: anti-microbial, anti-wrinkle, SPF-factor anti-carious, anti-inflammatory effects of oral hygiene products, etc.) certified by the applicant
- Extract from the Unified State Register of legal entities, or the Uniform State Register of Individual Entrepreneurs
References
- The Eurasian Economic Community, Customs Union Commission. The Technical Regulation on the Safety of Cosmetic Products. http://www.eurasiancommission.org/ru/Lists/EECDocs/P_799_3.pdf (August 2022)